Dual-Target SARS-CoV-2-qPCR for Covid-19 detection
More convenience, no false positive results*, high sensitivity
Covid-19 FluoGene Q kit is a CE-IVD marked real-time dual-target multiplex qRT-PCR for the qualitative detection of severe acute respiratory syndrome coronavirus (SARS-CoV-2) specific RNA.
*until 27th July 2024, no false positive results were observed by us or reported to us from our customers and partners
The isolated RNA target is transcribed into cDNA and the target genes are amplified in a single step qRT-PCR. The primer probe mixes detect the SARS-CoV-2 specific genes M and N1 , as well as the RNase P gene as an internal control reaction. A positive control is included in the test system.
The assay can be performed on any standard qPCR machine with channels for FAM, JOE and ROX.
The assay has been tested with RNA extracted from standard Viral Transfer Media (PBS) and with RNA extracted from GenXPros Rely buffer. For highest yield and more safety and sensitivity we strongly recommend to use the GenXPro Rely buffer for direct lysis of the swab-content.
Why Covid-19 FluoGene Q assay ?
…problems of the WHO E-gene assay and false positives
The highly sensitive E-gene specific primers of the WHO assay have been reported to generate up to 10% false positives which is why it is required to test the E-gene positive samples with a second qPCR assay.
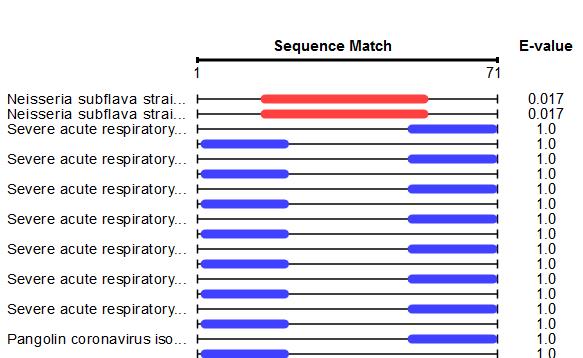
We sequenced a PCR product of a false positive sample and found a 70 bps long region matching to Neisseria subflava strains (see BLAST results of the amplicon below), a relatively common nasopharyngal bacterium.
The M-Gene Assay
Therefore, we adapted the M-gene assay (Toptan and Widera et al. 2020) for high specificity and potentially lower rates of false positives.
Samples that generated false positive results using the E-primers of the WHO/Charité assay were correctly found to be negative using our multiplex assays. The specificity was prior described by Toptan and Widera et al. 2020 and proven with samples that showed false positive results using E-Primers in our lab. Until 27th July 2024, no false positives have beeen observed in > 3000 in-house tests or were reported by our customers.
Please click below for further SARS-CoV-2 related products and Services
-
Simultaneous detection of SARS-CoV-2 and N501Y
-
E484K-specific qPCR Assay for SARS-CoV-2 Variants of Concern
-
RELY-viral medium: inactivating the virus, stabilizing the RNA at RT
-
Whole Genome Sequencing of SARS-CoV-2 for Surveillance (in German)
#Dual-target-SARS-CoV-2-triplex-assay
Contact
Please write us an e-mail (info@genxpro.de), or call us at (+49 69) 95739710 ).
Reference:
Optimized qRT-PCR approach for the detection of intra- and extra-cellular SARS-CoV-2 RNAs